I wanted to think of a very clever title for this epic topic, but I wanted to cut straight to the point. As the antibiotic era is coming to an end due to the emergence of antibiotic-resistant pathogens, it is alarming that we had not sufficiently explored the deleterious side effects that these drugs cause on our own cells.
James Collins and his group from the Howard Hughes Medical Institute published last week in Science Translational Medicine (my new favorite journal) a remarkable study that shows antibiotics not only harm bacterial cells, but mammalian cells too.

Let’s start in the 40’s…
Looking back in time, the term “antibiotics” was first used in 1942 by Selman Waksman to describe any substance synthesized by a microorganism that negatively affects the growth of other microorganisms. Antibiotics inhibit growth of microorganisms through a variety of mechanisms ranging from molecules that bind to ribosomal subunits to inhibit protein synthesis to disruption of bacterial lipid membranes.
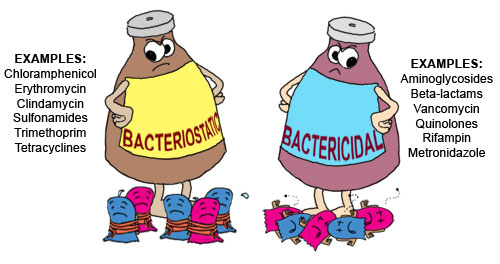
Generally all of the antibiotics can be categorized into two groups: 1) bactericidal antibiotics and 2) bacteriostatic antibiotics.
Bactericidal antibiotics directly kill bacteria (ie; penicillin, which blocks synthesis of the bacterial cell wall), whereas bacteriostatic antibiotics slow their growth or reproduction (ie; a macrolide like azithromycin, which binds to the 50S subunit
Interestingly, in 2007 James Collins and his group released a set of data that suggests that all bactericidal antibiotics have a common mechanism of killing bacteria. This blew our original knowledge of bactericidal antibiotics out the water. Here we’ve been thinking for nearly 7 decades that each individual class of bactericidal antibiotics kill through different mechanisms, but we were wrong? Perhaps (this hypothesis is highly debated, but should not be discounted!).
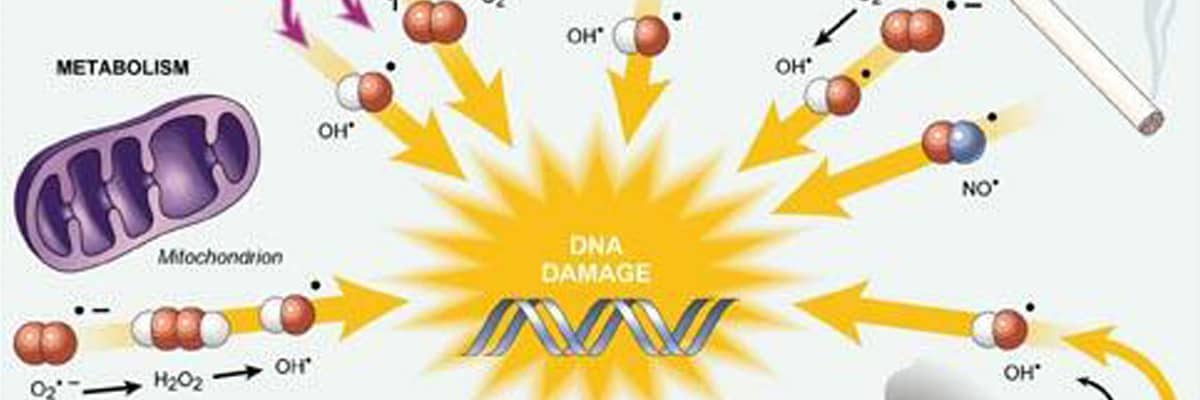
This study suggests that all bactericidal antibiotic killing starts out as an array of diverse disruption mechanisms to throw the bacteria out of homeostatis, but all of these mechanisms have a similar end point – production of “highly deleterious hydroxyl radicals in Gram-negative and Gram-positive bacteria, which ultimately contribute to cell death”.
Hydroxyl radicals are highly reactive ions that can damage virtually any type of molecule including DNA, lipids, amino acids… that’s just a taste of what these assassins can target and all three of those things have something in common… they are required for LIFE.
Not just bacterial life, ALL LIFE.
Ah ha!
So if production of hydroxyl radicals can have such a harmful affect on the essential essence of bacteria (DNA, proteins, amino acids, lipids, etc), couldn’t the production of hydroxyl radicals have an affect on these same essentials in mammalian cells?
Precisely!
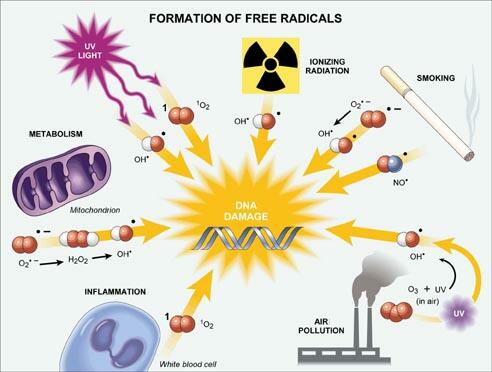
Interestingly prolonged use of antibiotics is associated with various side effects ranging from ototoxicity (damage to the ear due to use of antibiotics like aminoglycosides, which are antibiotics that are bactericidal in high concentrations) to nephrotoxicity (kidney disfunction). Despite these correlations, no real studies have been conducted on the adverse affects of bactericidal antibiotics on mammalian cells!
Turns out James Collins and his group show, after intense investigation into this matter, that bactericidal antibiotics (specifically quinolones, aminoglycosides, and beta-lactams) can cause mitochondrial dysfunction and reactive oxygen species (ROS) overproduction in human and mice cells.
Turns out hydroxyl radicals are a type of ROS. Whoa mama. Ultimately the over-production of ROS induced damage to DNA, proteins, and membrane lipids leading to tissue damage in the host.
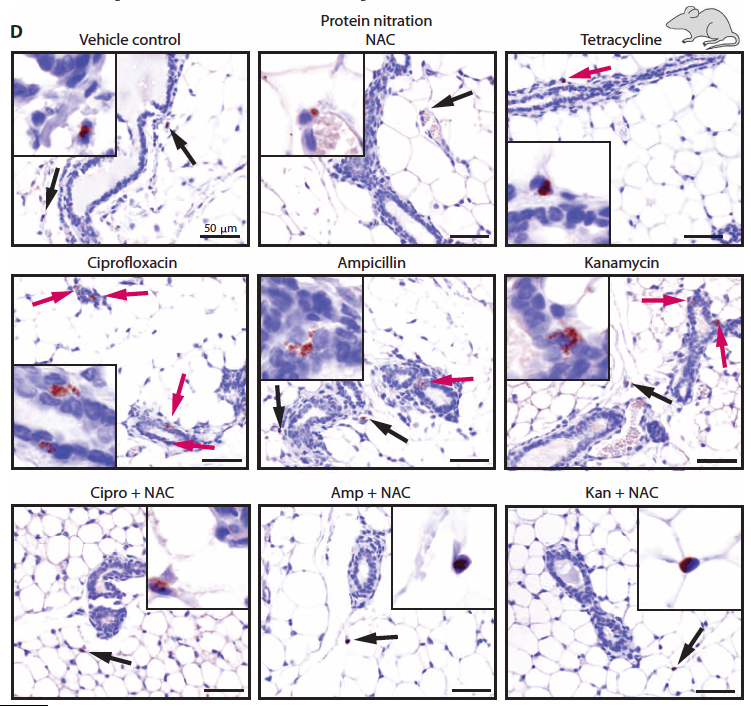
Amazing, but somewhat intuitive, if they gave mice the Food and Drug Administration (FDA)-approved antioxidant (think pomegranates, or don’t apparently that’s not scientifically proven), N-acetyl-L-cysteine (NAC), it completely alleviated all of the scary side affects of these bactericidal antibiotics.
There is huge implication for this study on long-term antibiotic treatment or treatment of antibiotic cocktails in the ICU (mostly immune suppressed patients) in which antibiotic regiments may have to be revised – perhaps by supplementing patients afternoon sandwiches with some anti-oxidant containing blueberries?