-

Treatment with antibiotics frees sugars used by pathogens
•
We have tiny visitors that have taken advantage of their evolutionary squatting rights throughout our bodies; these microbes make up our microbiome. Nobel Laureate Joshua Lederberg best defined the human microbiome as “the ecological community of commensal, symbiotic, and pathogenic microorganisms that literally share our body space”. We are Soylent…
-

Image-focused: This week in astronomy
•
Astronomers collect light. That’s all they do. They make it into pictures and scatter plots (and histograms and spectra and tables), but everything they figure out comes from photons with different energies, wavelengths, orientations, origins, and hair colors. In celebration of that fact, it’s Friday Picture Day. The four images…
-
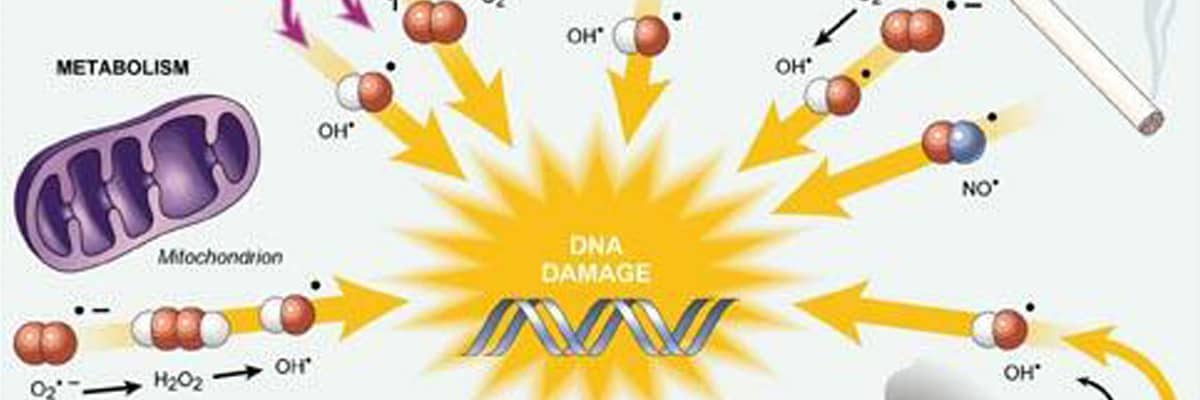
Antibiotics damage human cells
•
I wanted to think of a very clever title for this epic topic, but I wanted to cut straight to the point. As the antibiotic era is coming to an end due to the emergence of antibiotic-resistant pathogens, it is alarming that we had not sufficiently explored the deleterious side…
-
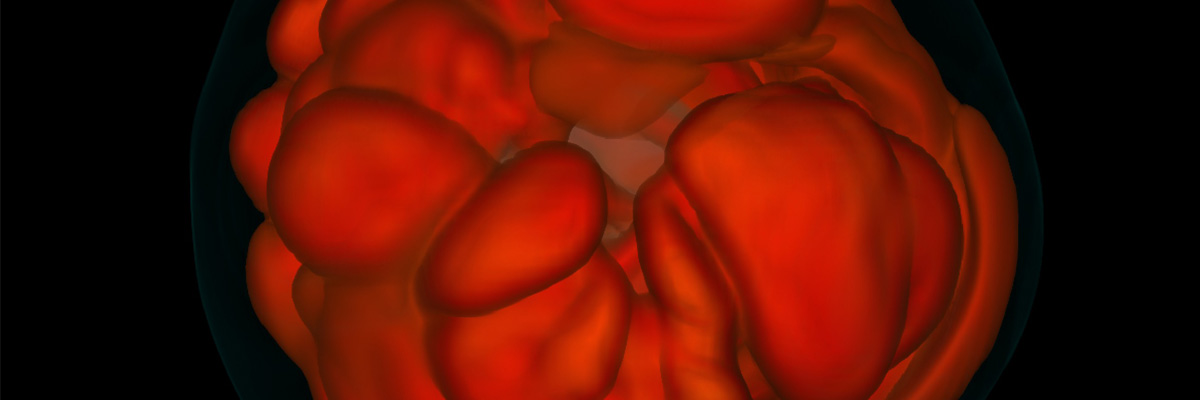
Sassy supernovae: A new 3D simulation shows dying stars slosh
•
It’s no secret that when a massive star dies, it often does so dramatically–in a huge explosion known as a supernova. Supernovae outshine entire galaxies and enrich the universe with the heavy elements that are necessary to make those gold chains you wear. It is kind of a secret, though,…
-

What’s Closer Than Moon
•
Unlocking the Secrets of Asteroid YU55: A Deep Dive with the Green Bank Telescope In an ambitious endeavour to peel back the layers of cosmic mysteries, Dr. Michael Busch and his team have turned their sights toward asteroid YU55, utilizing the formidable Green Bank Telescope (GBT) to explore what lies…
-

Well I’m Back To Regular Life
•
From Fields to Cityscapes: A Scientist’s Journey Welcome to my blog! Today, I want to share the extraordinary journey of a scientist whose world is rooted deeply in the serene setting of a farm, amidst the whispers of plants and the secrets of nature. Let’s go on this intriguing voyage…
